Modified True/False
Indicate
whether the statement is true or false. If false, change the identified word or phrase to make the
statement true.
|
|
|
1.
|
The freezing of water is a chemical change.
_________________________
|
|
|
2.
|
The color of a substance is a physical property.
_________________________
|
|
|
3.
|
Homogeneous mixtures include colloids, solutions, and suspensions.
_________________________
|
|
|
4.
|
Smoke is a compound. _________________________
|
|
|
5.
|
salt water is a homogeneous mixture. _________________________
|
|
|
6.
|
Pure substances are either elements or mixtures.
_________________________
|
Matching
|
|
|
Match each term with the correct definitions below. a. | element | d. | compound | b. | mixture | e. | matter | c. | substance |
|
|
|
7.
|
composition is different throughout
|
|
|
8.
|
all atoms the same
|
|
|
9.
|
has mass, takes up space
|
|
|
10.
|
two or more elements combined chemically
|
|
|
11.
|
composition stays the same
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
12.
|
One example of a physical change is
a. | burning paper. | c. | baking cookies. | b. | the rusting of iron. | d. | mixing a
milkshake. |
|
|
|
13.
|
What is one difference between a mixture and a compound?
a. | A compound consists of more than one phase. | b. | A mixture can only
be separated into its components by chemical means. | c. | A mixture must be uniform in
composition. | d. | A compound can only be separated into its components by chemical
means. |
|
|
|
14.
|
Substances that CANNOT be broken down chemically into other substances
are
a. | compounds. | b. | elements. | c. | mixtures. | d. | liquids. |
|
|
|
15.
|
Which of the following can be classified as a mixture?
a. | pure air | b. | pure water | c. | pure gold | d. | pure
nitrogen |
|
|
|
16.
|
When a physical change in a sample occurs, which of the following does NOT
change?
a. | composition | c. | temperature | b. | shape | d. | volume |
|
|
|
17.
|
Which of the following is a physical change?
a. | explosion | b. | rotting of food | c. | corrosion | d. | evaporation |
|
|
|
18.
|
Which type of matter consists of two or more substances that are NOT chemically
combined?
a. | mixtures | b. | elements | c. | atoms | d. | compounds |
|
|
|
19.
|
In chemistry, elements are represented by
a. | symbols. | b. | building blocks. | c. | ratios. | d. | formulas. |
|
|
|
20.
|
Which of the following is a heterogeneous mixture?
a. | sugar water | c. | stainless steel | b. | water in a swimming pool | d. | a jar of mixed
nuts |
|
|
|
21.
|
Which of the following has the highest viscosity?
a. | orange juice | c. | corn syrup | b. | milk | d. | water |
|
|
|
22.
|
A mixture that appears to contain only one substance is a(an)
a. | element. | c. | compound. | b. | heterogeneous mixture. | d. | homogeneous
mixture. |
|
|
|
23.
|
The measurement of how much matter an object contains is its
a. | volume. | b. | density. | c. | mass. | d. | weight. |
|
|
|
24.
|
____ is another name for a homogeneous mixture.
a. | Liquid | b. | Solution | c. | Suspension | d. | Substance |
|
|
|
25.
|
Which of the following is NOT an example of matter?
a. | water vapor | b. | smoke | c. | air | d. | heat |
|
|
|
26.
|
Which of the following is malleable?
a. | glass | b. | gold | c. | ice | d. | pottery |
|
|
|
27.
|
Which of the following is a characteristic of a mixture?
a. | has varying properties | c. | contains only pure substances | b. | has a fixed
composition | d. | both a and
b |
|
|
|
28.
|
Which of the following substances is an element?
a. | Iron | b. | Salt | c. | Sugar | d. | Baking soda |
|
|
|
29.
|
All of the following are examples of properties EXCEPT
a. | symbols. | b. | color. | c. | flammability. | d. | hardness. |
|
|
|
30.
|
Which of the following is a clue of a chemical change?
a. | balls of wax form when melted wax is poured into ice water | b. | gas bubbles form in
boiling water | c. | a gas forms when vinegar and baking soda are mixed | d. | iron changes color
when heated |
|
|
|
31.
|
When two or more substances are combined so each substance can be
separated by physical means, the result is a(n)____.
a. | chemical change | c. | compound | b. | mixture | d. | element |
|
|
|
32.
|
Which of the following is a physical change?
a. | burning a piece of wood | b. | a copper roof changing color from red to
green | c. | sawing a piece of wood in half | d. | rust forming on an iron
fence |
|
|
|
33.
|
The first letter in a properly written chemical symbol is always
a. | bold faced. | b. | capitalized. | c. | italicized. | d. | underlined. |
|
|
|
34.
|
What holds atoms together in a molecule?
a. | gravity | b. | chemical bonds | c. | temperature | d. | density |
|
|
|
35.
|
One example of a chemical change is
a. | cutting up paper. | c. | boiling water. | b. | crushing a can. | d. | burning gasoline in an
engine. |
|
|
|
36.
|
Which of the following is NOT a pure substance?
a. | carbon dioxide | b. | water | c. | pizza | d. | oxygen |
|
|
|
37.
|
How many Chlorine atoms make up one unit of aluminum chloride,
AlCl3?
|
|
|
38.
|
Which of the following is a physical property?
a. | explosive | b. | ability to rust | c. | combustible | d. | melting
point |
|
|
|
39.
|
Which is the type of separation technique being demonstrated in the
figure? 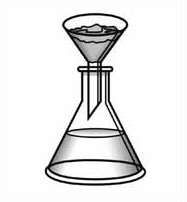 a. | chromatography | c. | filtration | b. | distillation | d. | crystallization |
|
|
|
40.
|
All elements are composed of extremely small particles called
a. | atoms. | b. | compounds. | c. | mixtures. | d. | molecules. |
|